Definitions:
Acid: Acid is the substance whose solution turns blue litmus paper red. They have a sour taste.
Base: It is substance whose solution turns red litmus paper blue. They have a bitter taste and their solutions feel slippery like soap water.
Acidimetry: It is acid base titration method used to determine concentration of basic substances by titration with a standard base solution.
Alkalimetry: It is acid base titration method used to determine concentration of basic substances by titration with a standard acid.
Titrant: A substances or a reagent solution of precisely known concentration that is added in titration. It is generally filled in burette.
Titer: A substance which is being analyzed in the titration, it is generally taken in reaction flask.
Titration: The process, operation, or method used for finding out the concentration of substance in solution by adding to it a standard reagent of known concentration until reaction is complete.
End point: It is the point where the titration ends in practice, the point when the indicator color changes.
Equivalence point: The point when the number of equivalents mixed together is the same. The Equivalence point is where the reaction is theoretically complete.
Neutralization: A chemical reaction where an acid is reacted with an equivalent amount of base.
Titration Curve: Plot the pH of the solution as a function of the volume of titrant added.
Indicators: An auxiliary chemical compound that changes color and structure when exposed to certain conditions and therefore uses to detect the end point of the titration.
Theories of Acids and Bases:
The Arrhenius Theory:
- Some compounds are basic in nature but they do not contain hydroxyl group(OH–) in its structure.
- Some of the compound do not contain hydrogen ions but still shows acidic property in aqueous medium, example : FeCl3
- The Arrhenius acid base concept is applicable in aqueous state but not for the gaseous state.
- Arrhenius theory says that H+ available freely in aqueous solution, but essentially H+ is always hydrated to form hydronium ion (H₃O⁺)
The Bronsted-Lowry Theory
- This theory could not explain the acid-base behavior in aprotic solvents like Benzene(
C6H6 ), etc. - Since there is no proton transfer reaction between acid oxides, for example, Sulphur dioxide and Carbon dioxide respectively and basic oxides for example Calcium oxide(CaO)
and Magnesium oxide(MgO) respectively. It failed to explain this type of reaction. - It failed to exhibit the acidic nature of substances like Aluminium chloride(
AlCl3 ) and Boron trifluoride(BF₃) with no proton. - Here, water(
H₂O ) is acting as Bronsted Lowry acid and Ammonia(NH3 ) is acting as Bronsted Lowry's base.
The Lewis Reaction
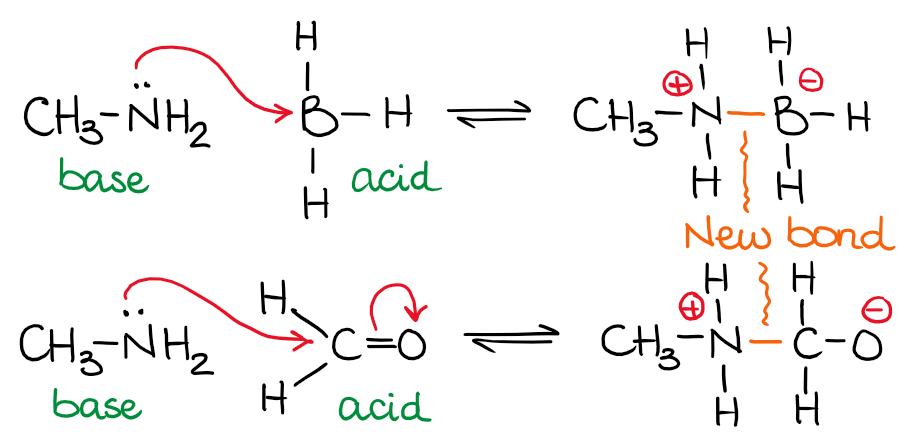
Limitations of Lewis concept:
- The relative strength of acids and bases can no be explained by this concept.
Theories of Acids and base indicator:-
- Ostwald's Theory: In 1894,Ostawald proposed the theory of indicator on the basis of ionization. According to this theory, the color change is due to ionization of the acid-base indicator. The unionized from has different color than the ionized form. The ionization of the indicator is largely affected in acids and bases as it is either a weak acid or a weak base. In case, the indicator is a weak acid, its ionization is very much low in acids, due to the common H+ ions while it is fairly ionized in alkalis. Example: Considering phenolphthalein in an important indicator, Ostwald theory can be illustrated as follows:- The undissociated molecules of phenolphthalein are colorless while Ph- ions are pink in colour.
- An acid-base indicator is usually a weak organic acid or base.
- If the indicator is weak acid, the color of its anions will be deeper than that of unionized form.
- Although acid-base indicators are weak electrolytes.
- Quinonoid theory:



Salient Feature:
- Each acid-base indicator exists in two or more tautomeric forms, one in acidic medium and the other in alkaline solution.
- Colours of the two tautomeric forms are different due to the difference in their structures.







0 Comments
Please do not enter any spam link in the comment box