Emulsions:
They are the biphasic liquid preparation containing two immiscible liquids, one of which is dispersed as minute globules into the other. The liquid which is converted to minute globules are called as dispersed phase and liquid in which globules are dispersed is called as continuous phase.
Stability problems of emulsions and methods to overcome
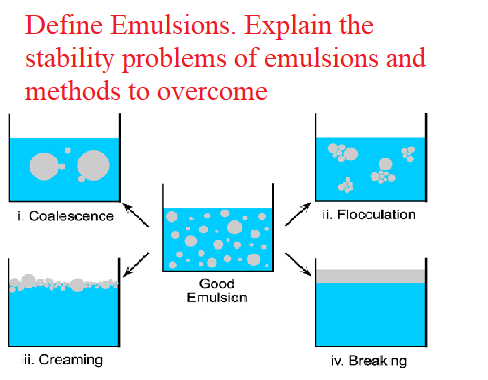
A stable emulsion is one in which the globules retain their initial character like mean size and size distribution and remain uniformly distributed throughout the continuous phase. During storage of emulsions instability is endorsed by cracking, reversible aggregation and/or irreversible aggregation etc.
a) Cracking or coalescence:
- Coalescence is a growth process during which the emulsified particles join to from large particles.
- When small droplets are merged and large droplets are fed, it suggests that the emulsion will separate completely and cannot be re-dispersed by shaking.
- This process is also known as cracking. Any chemical, physical or biological effects that changes the nature of the interfacial film of an emulsifying agent may cause cracking. Creaming can be induced by the following methods.
- Addition of an emulsifying agent of opposite type
- Decomposition or precipitation of emulsifying agent.
- Addition of a common solvent.
- Addition of a proper preservatives
- Incorporation of excess of disperse h.
- Under the influence of gravity suspended particles or globules tend to upward movement, known as creaming while downward movement of particles or droplets is called sedimentation.
- Creaming or sedimentation depends on the differences in specific gravity between the phases. If creaming take place without any aggregation, the emulsion can be reconstituted by shaking. process of creaming is explained by Stoke's law.
- Globules size: Globules of small size have less tendency to cream.
- Viscosity: Higher the viscosity of continuous phase, less creaming.
- Density: Less difference in density of two phases means more stability of emulsion.
- By the addition of an electrolyte.
- By changing the phase-volume ratio
- By temperature change
- By changing the emulsifying agent.





0 Comments
Please do not enter any spam link in the comment box