
Electrochemical cell
Electrodes used in Potentiometry
- Reference Electrode
- Indicator Electrode
i)Reference Electrode
- The potential of reference electrode is known and has constant value i.e. it is independent of analyte composition.
- Primary Electrodes : a)Hydrogen Electrodes
- Secondary Electrodes : a)Saturated calomel Electrodes
a)Standard Hydrogen Electrode
Standard Hydrogen Electrode is used as a reference electrode when calculating the standard electrode potential of a half cell.
Construction
The parts that make up a Standard Hydrogen Electrode are listed below.
- A platinum electrode which is covered in finely powdered platinum black (platinized platinum electrode).
- A hydrogen Blow.
- A solution of acid having a H+ molarity of 1 mole per cubic decimeter.
- The SHE also contains a hydro seal which is used to prevent the interference of oxygen.
- The other half-cell of the entire Galvanic cell must be attached to the Standard Hydrogen Electrode through a reservoir in order to create an ironically conductive path. This can be done through a direct connection, through a narrow tube, or even through the use of a salt bridge.
Diagram
Advantages
1.It gives reproducible result.
2.It has no salt error.
3.It can be used over entire pH range.
4.It is used as a fundamental electrode.
5.It can be used as both indicator electrode as well as reference electrode.
Disadvantages
- It is not useful in solutions containing strong oxidizing or reducing agent.
- It is not useful in solution having metal ions that are below Hydrogen in potential Series.
Use of Platinum in the Standard Hydrogen Electrode
Platinum is used in the Standard Hydrogen Electrode due to the following reasons:
- Platinum is a relatively inert metal which does not corrode easily.
- Platinum has catalytic qualities which promotes the proton reduction reaction.
- The surface of platinum can be covered with platinum black, a fine powder of platinum. This type of platinum electrode is called a platinized platinum electrode.
- Platinum also improves the reaction kinetics by adsorbing hydrogen at the interface.
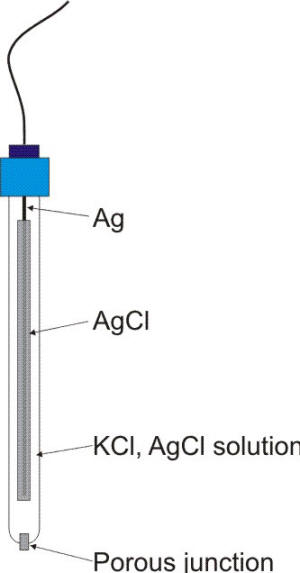
b)Silver-Silver Chloride Electrode
The silver/silver chloride reference electrode is a widely used reference electrode because it is simple, inexpensive, very stable and non-toxic. As a laboratory electrode such as described in the following Figure, it is mainly used with saturated potassium chloride (KCl) electrolyte, but can be used with lower concentrations such as 1 M KCl and even directly in seawater.
Construction
- It is simply a silver wire coated electrolytically with silver chloride and dipped into potassium chloride
- The potential of this half cell also depends upon temperature as well concentration of KCl used
Diagram
Advantage
- Easy to use
Disadvantage
- Difficult of prepare
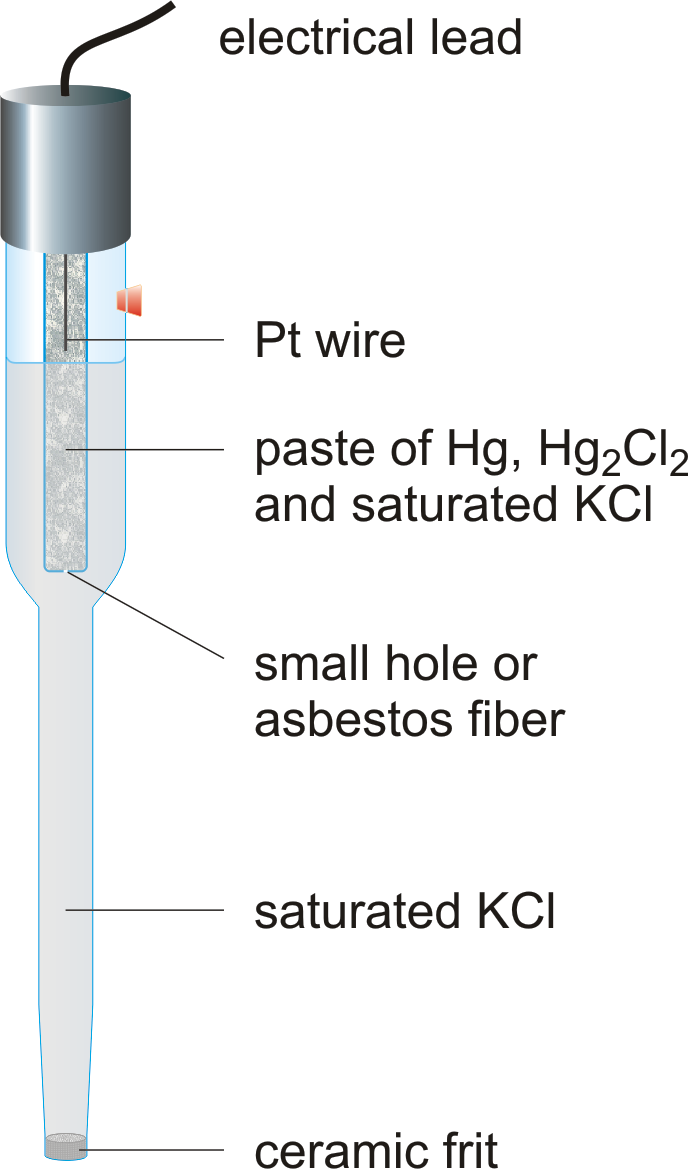
c)Saturated calomel electrode
The Saturated calomel electrode (SCE) is a reference electrode based on the reaction between elemental mercury and mercury(I) chloride. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel") is a saturated solution of potassium chloride in water. The electrode is normally linked via a porous frit to the solution in which the other electrode is immersed. This porous frit is a salt bridge.
Construction
- It consist of an inner jacket and outer sleeve
- The inner tube(jacket)has has wire which is connected with mercury and plugged with a mixture of calomel and KCl
- The outer tube is surrounded by an outer sleeve and the tip is sealed with crystal or KCl and poroces plug of asbestos.
- The space between the inner jacket and outer sleeve is filled with either saturated KCl or 1N KCl or 0.1Kcl on which the potential of the electrode depends
- The potential of this half cell depends upon concentration of KCl used and upon concentration of KCl used.
Diagram
Theory of operation
The electrode is based on the redox reaction
The Nernst equation for this reaction is
where E0 is the standard electrode potential for the reaction and aHg is the activity for the mercury cation (the activity for a liquid is 1). This activity can be found from the solubility product of the reaction
By replacing the activity in the Nernst equation with the value in the solubility equation, we get
The only variable in this equation is the activity (or concentration) of the chloride anion. But since the inner solution is satured with potassium chloride, this activity is fixed by the solubility of potassium chloride. At standard conditions, the potential of the saturated calomel electrode should be +0.241 V versus the SHE.
Application
The SCE is used in pH measurement, cyclic voltammetry and general aqueous electrochemistry.
This electrode and the silver/silver chloride reference electrode work in the same way. In both electrodes, the activity of the metal ion is fixed by the solubility of the metal salt.
The calomel electrode contains mercury, which poses much greater health hazards than the silver metal used in the Ag/AgCl electrode
Advantages
- Eare of Constraction
- Stability of potential
Disadvantages
- Unstable at Temparature a bore 80c
- Not Suitable when chloride ions show incompatibility
ii) Indicator electrodes
a)Glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of ph. The pH electrode is an example of a glass electrode that is sensitive to hydrogen ions. Glass electrodes play an important part in the instrumentation for chemical analysis and physico-chemical studies. The voltage of the glass electrode, relative to some reference value, is sensitive to changes in the activity of certain type of ions.
Construction
- Glass electrode most widely used indicator electrode
- It is selective and responsive to change in concentration of H+ ions
- glass electrode consists of a glass tube with a thin pH sensitive glass bulb at its tip
- It has a silver-silver chloride wire at the center of the tube and the lower tip of the wire is dipped into the 0.1N HCl filled in the glass tube .This assembly acts as an indicator electrode and is dipped into a solution whose pH or potential is to be unknown.
- glass bulb acts like a semipermeable membrane for ions .the H+ ions egarily enter the glass
Diagram
Advantages
- It gives a rapid response.
- It is simple to operate.
- It can be used in viscous colored solution
- It is no salt error
Disadvantage
- It is fragile, So should be handled carefully
- Minute scratches make glass electrodes useless
- It is must be hydrated all the time
- It is not useful in highly alkaline solution as there occurs partial exchange of cations than H+ ions
Applications
- Glass electrodes are commonly used for pH measurements.
- There are also specialized ion sensitive glass electrodes used for determination of the concentration of lithium, sodium, ammonium, and other ions.
- Glass electrodes have been utilized in a wide range of applications including pure research, control of industrial processes, analysis of foods and cosmetics, measurement of environmental indicators, and microelectrode measurements such as cell membrane electrical potential and soil acidity.
Methods of Determine end point
- There are various methods to locate the end point.
- The main purpose to to know the end-point at which the quantities of reaching species are present in equal amount.
- The equivalent point is generally found graphically
- There are following methods for calculating end point:-
- Value of EMF or pH are recorded after addition of each volume of titrant.
- Then a graph is plotted between volume of titrant added v/s values of pH or EMF.
- At the equivalent point there is a sharp increase in potential or there is maximum slop of curve.
- Generally S-shaped curve is obtained.

- Difference of EMF or pH for the volume of titrant added is plotted against(V) added .
- A bell shaped graph is obtained and end point there is maximum charge in EMF or pH .
- The end point can be recorded by drawing perpendicular from peak of graph on volume excess.

- ∆²E/∆v² against the volume of titrant added is pointed .
- The end point can be obtained ass a zero point where the slope curve of ∆²E/∆v² is maximum



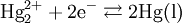
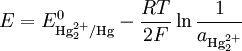
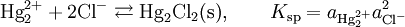
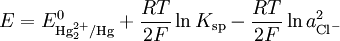




0 Comments
Please do not enter any spam link in the comment box