- A compound is said to be impure if it is having foreign matter i.e. impurities
- impurities is define as the foreign particle that affect the purity of a substance .
Source of Impurities
1.Raw material used in pharmaceutical preparation:
2.Methode of Manufacture:
a)Reagents used in starting material:
b)Reagents used to remove other impurity:
c)Solvent :
- Tap Water : It contain Ca++,Mg²++,Na++,Cl-,So₄-² &Co₃²𐄐 as impurities in very small amounts.
- Softened water: It is obtain by allowing tap water to pass through the sodium from of zeolite which removes divalent cations ( Ca++,Mg²++) but still other impurities will be present.
- Demineralized water: By this process ,all minerals are removed but still few organic materials may be present they act as impurity.
- Distilled Water: It is considered to be beat because it is free from all inorganic and organic impurities and it is pure
- Reaction Vessel: During the manufacturing process, sometimes the solvents or reagent may under go chemical reaction with the vessel and contributes to impurities in the end product .
3.Intermediates:
4.Atomospheric condition
5.Manufacturing Hazards:
- Contamination due to particular matter: The presence of unwanted particulate matter like dust, glass, metallic ions, porcelain etc. can arise in a number of ways, causing contamination of the pharmaceutical items.
- Cross-Contamination: The handling of powders granules and tablet in large-bulk frequently creates a considerable amount of air borne. dust ,which if not controlled can lead to cross-contamination of products.
- Microbial contamination: Generally products like creams, topical application to broken skin or mucous membranes are liable to bacterial mould and fungal controlled either by doing sterlized test or by other methods.
- Error during manufacturing process: During the manufacturing of an item due to use of incorrect quantities of reagent or even solvent or due to incorrect methods of mixing filling tableting the outer can be hazardous.
- Packaging errors/Errors during package: To similar shape, colour and storing vessels ,sometimes there can be mistake while packing the drug and may lead to critical or hazards outcome if used wrongly by a patient.
6.Storage condition:
- Chemical instability of the product: The drugs or medicine of final product may get disturbed and undergo chemical change. Usually it occurs due to light ,air, humidity or temperature.
- Physical changes: the occurrence of change in physical form of the drug during storage is not unknown changes in crystal size and form agglomeration may occur with no changes in chemical properties .which make the drugs hazardous
- Reaction with the container: Pharmaceutical preparation when stored in inappropriate containers react with the material of the container and undergo deterioration . e.g. salicylic acid ointment shouldn't be packed in metal containers .atropine sulphate injection should not be stored in sod.
- Temperature: The rate at which chemical and also physical changes occurs in stored products is conditioned by temperature and hence the changes in temperature may trigger the change and cause the product to be harmful.
Effect of Impurities
2 Impurities may cause difficulties during formulations and use of the substance .
3 Impurities may lower the shelf life of the substance
4 Sometimes impurities changes the physical and chemical properties of the substances
5 Therapeutic effect can be decreased
6 shows toxic effect after a certain period
7 injurious when present above certain limits .
8 It may changes odor ,colour, taste of the substance
Principle involved in Limit test for:
Limit Test of Chloride
Principle:
Limit
test of chloride is based on the reaction of soluble chloride with
silver nitrate in presence of dilute nitric acid to form silver
chloride, which appears as solid particles (Opalescence) in the
solution.
AgNO3(aq)+NaCl(aq)→AgCl(s)+NaNO3(aq) Procedure:
Test sample
Standard compound
Specific
weight of compound is dissolved in water or solution is prepared as
directed in the pharmacopoeia and transferred in Nessler cylinder
Take 1ml of 0.05845 % W/V solution of sodium chloride in Nessler cylinder
Add 1ml of nitric acid
Add 1ml of nitric acid
Dilute to 50ml in Nessler cylinder
Dilute to 50ml in Nessler cylinder
Add 1ml of AgNO3 solution
Add 1ml of AgNO3 solution
Keep aside for 5 min
Keep aside for 5 min
Observe the Opalescence/Turbidity
Observe the Opalescence/Turbidity
Observation:
The
opalescence produce in sample solution should not be greater than
standard solution. If opalescence produces in sample solution is less
than the standard solution, the sample will pass the limit test of
chloride and visa versa.
Reasons:
Nitric
acid is added in the limit test of chloride to make solution acidic and
helps silver chloride precipitate to make solution turbid at the end of
process.
Limit test of chloride is based on the reaction of soluble chloride with silver nitrate in presence of dilute nitric acid to form silver chloride, which appears as solid particles (Opalescence) in the solution.
| Test sample | Standard compound |
|---|---|
| Specific weight of compound is dissolved in water or solution is prepared as directed in the pharmacopoeia and transferred in Nessler cylinder |
Take 1ml of 0.05845 % W/V solution of sodium chloride in Nessler cylinder |
| Add 1ml of nitric acid | Add 1ml of nitric acid |
| Dilute to 50ml in Nessler cylinder | Dilute to 50ml in Nessler cylinder |
| Add 1ml of AgNO3 solution | Add 1ml of AgNO3 solution |
| Keep aside for 5 min | Keep aside for 5 min |
| Observe the Opalescence/Turbidity | Observe the Opalescence/Turbidity |
The opalescence produce in sample solution should not be greater than standard solution. If opalescence produces in sample solution is less than the standard solution, the sample will pass the limit test of chloride and visa versa.
Reasons:
Nitric acid is added in the limit test of chloride to make solution acidic and helps silver chloride precipitate to make solution turbid at the end of process.
Limit Test of Iron
Principle:
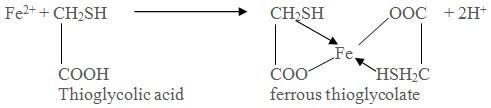
Limit
test of Iron is based on the reaction of iron in ammonical solution
with thioglycollic acid in presence of citric acid to form iron
thioglycolate which is pale pink to deep reddish purple in color.
Test sample
Standard compound
Sample is dissolved in specific amount of water and then volume is made up to 40 ml
2 ml of standard solution of iron diluted with water upto 40ml
Add 2 ml of 20 % w/v of citric acid (iron free)
Add 2 ml of 20 % w/v of citric acid (iron free)
Add 2 drops of thioglycollic acid
Add 2 drops of thioglycollic acid
Add ammonia to make the solution alkaline and adjust the volume to 50 ml
Add ammonia to make the solution alkaline and adjust the volume to 50 ml
Keep aside for 5 min
Keep aside for 5 min
Color developed is viewed vertically and compared with standard solution
Color developed is viewed vertically and compared with standard solution
Earlier
aamonium thiocyanate reagent was used for the limit test of iron. Since
thioglycolic acid is more sensitive reagent, it has replaced ammonium
thiocyanate in the test.
Observation:
The
purple color produce in sample solution should not be greater than
standard solution. If purple color produces in sample solution is less
than the standard solution, the sample will pass the limit test of iron
and vice versa.
Reasons:
Citric acid helps precipitation of iron by ammonia by forming a complex with it.
Thioglycolic acid helps to oxidize iron (II) to iron (III).
Ammonia to make solution alkalin
Principle:
Limit test of Iron is based on the reaction of iron in ammonical solution with thioglycollic acid in presence of citric acid to form iron thioglycolate which is pale pink to deep reddish purple in color.
| Test sample | Standard compound |
|---|---|
| Sample is dissolved in specific amount of water and then volume is made up to 40 ml | 2 ml of standard solution of iron diluted with water upto 40ml |
| Add 2 ml of 20 % w/v of citric acid (iron free) | Add 2 ml of 20 % w/v of citric acid (iron free) |
| Add 2 drops of thioglycollic acid | Add 2 drops of thioglycollic acid |
| Add ammonia to make the solution alkaline and adjust the volume to 50 ml | Add ammonia to make the solution alkaline and adjust the volume to 50 ml |
| Keep aside for 5 min | Keep aside for 5 min |
| Color developed is viewed vertically and compared with standard solution | Color developed is viewed vertically and compared with standard solution |
Earlier aamonium thiocyanate reagent was used for the limit test of iron. Since thioglycolic acid is more sensitive reagent, it has replaced ammonium thiocyanate in the test.
Limit Test of Sulphate
Principle:
Limit
test of sulphate is based on the reaction of soluble sulphate with
barium chloride in presence of dilute hydrochloric acid to form barium
sulphate which appears as solid particles (turbidity) in the solution

Test sample
Standard compound
Specific weight of compound is dissolved in water or solution is prepared as directed in the pharmacopoeia and transferred in Nessler cylinder
Take 1ml of 0.1089 % W/V solution of potassium sulphate in Nessler cylinder
Add 2ml of dilute hydrochloric acid
Add 2ml of dilute hydrochloric acid
Dilute to 45 ml in Nessler cylinder
Dilute to 45 ml in Nessler cylinder
Add 5ml of barium sulphate reagent
Add 5ml of barium sulphate reagent
Keep aside for 5 min
Keep aside for 5 min
Observe the Turbidity
Observe the Turbidity
Barium sulphate reagent contains barium chloride, sulphate free alcohol and small amount of potassium sulphate.
Observation:
The
turbidity produce in sample solution should not be greater than
standard solution. If turbidity produces in sample solution is less than
the standard solution, the sample will pass the limit test of sulphate
and vice versa.
Reasons:
Hydrochloric acid helps to make solution acidic.
Potassium sulphate is used to increase the sensitivity of the test by giving ionic concentration in the reagent
Alcohol helps to prevent super saturation.
Limit test of sulphate is based on the reaction of soluble sulphate with barium chloride in presence of dilute hydrochloric acid to form barium sulphate which appears as solid particles (turbidity) in the solution
| Test sample | Standard compound |
|---|---|
| Specific weight of compound is dissolved in water or solution is prepared as directed in the pharmacopoeia and transferred in Nessler cylinder | Take 1ml of 0.1089 % W/V solution of potassium sulphate in Nessler cylinder |
| Add 2ml of dilute hydrochloric acid | Add 2ml of dilute hydrochloric acid |
| Dilute to 45 ml in Nessler cylinder | Dilute to 45 ml in Nessler cylinder |
| Add 5ml of barium sulphate reagent | Add 5ml of barium sulphate reagent |
| Keep aside for 5 min | Keep aside for 5 min |
| Observe the Turbidity | Observe the Turbidity |
Observation:
The turbidity produce in sample solution should not be greater than standard solution. If turbidity produces in sample solution is less than the standard solution, the sample will pass the limit test of sulphate and vice versa.
Reasons:
Hydrochloric acid helps to make solution acidic.
Potassium sulphate is used to increase the sensitivity of the test by giving ionic concentration in the reagent
Alcohol helps to prevent super saturation.
Limit Test of Arsenic
Principle:
Limit test of Arsenic is based on the
reaction of arsenic gas with hydrogen ion to form yellow stain on
mercuric chloride paper in presence of reducing agents like potassium
iodide. It is also called as Gutzeit test and requires special
apparatus.
Arsenic, present as arsenic acid in the sample is reduced to arsenious
acid by reducing agents like potassium iodide, stannous acid, zinc,
hydrochloric acid, etc. Arsenious acid is further reduced to arsine
(gas) by hydrogen and reacts with mercuric chloride paper to give a
yellow stain.
H3AsO4 + H2SnO2 → H3AsO3 + H2SnO3
Arsenic acid Arsenious acid
H3AsO3 + 3H2 → AsH3 + 3H2O
Arsenious acid Arsine
The depth of yellow stain on mercuric chloride paper will depend upon the quality of arsenic present in the sample.
Procedure:
Test solution:
The test solution is prepared by dissolving
specific amount in water and stannated HCl (arsenic free) and kept in a
wide mouthed bottle.
To this solution 1 gm of KI, 5 ml of stannous chloride acid solution and
10 gm of zinc is added (all this reagents must be arsenic free)
Keep the solution aside for 40 min and stain obtained on mercuric chloride paper is compared with standard solution.
Standard solution:
A known quantity of dilute arsenic solution
is kept in wide mouthed bottle and rest procedure is followed as
described in test solution.
A : approximately 60 ml generator bottle with 40 ml indicating line.

B : glass tube with 6.5 mm inner diameter
C and D : a ground joint glass tube with 6.5 mm inner diameter and 18 mm
outer diameter at the joint. Inner joint and the outer joint form a
concentric circle.
E : rubber stopper
F : narrow part of the glass tube B. Glass wool is inserted up to this part.
G : rubber board (Lead acetate cotton plug)
H : clamp
Reasons:
Stannous chloride is used for complete evolution of arsine
Zinc, potassium iodide and stannous chloride is used as a reducing aged
Hydrochloride acid is used to make the solution acidic
Lead acetate pledger or papers are used to trap any hydrogen sulphide which may be evolved along with arsine.

Limit Test of Lead
Lead
is a most undesirable impurity in medical compounds and comes through
use of sulphuric acid, lead lined apparatus and glass bottles use for
storage of chemicals.
Principle:
Limit
test of lead is based on the reaction of lead and diphenyl thiocabazone
(dithizone) in alkaline solution to form lead dithizone complex which
is read in color.
Dithizone is green in color in chloroform and lead-dithizone complex is
violet in color, so the resulting color at the end of process is red.
Test sample
Standard compound
A known quantity of sample solution is transferred in a separating funnel
A standard lead solution is prepared equivalent to the amount of lead permitted in the sample under examination
Add 6ml of ammonium citrate
Add 6ml of ammonium citrate
Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride
Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride
Add 2 drops of phenol red
Add 2 drops of phenol red
Make solution alkaline by adding ammonia solution.
Make solution alkaline by adding ammonia solution.
Extract with 5 ml of dithizone until it becomes green
Extract with 5 ml of dithizone until it becomes green
Combine dithizone extracts are shaken for 30 mins with 30 ml of nitric acid and the chloroform layer is discarded
Combine dithizone extracts are shaken for 30 mins with 30 ml of nitric acid and the chloroform layer is discarded
To the acid solution add 5 ml of standard dithizone solution
To the acid solution add 5 ml of standard dithizone solution
Add 4 ml of ammonium cyanide
Add 4 ml of ammonium cyanide
Shake for 30 mins
Shake for 30 mins
Observe the color
Observe the color
Observation:
The
intensity of the color of complex, is depends on the amount of lead in
the solution. The color produce in sample solution should not be greater
than standard solution. If color produces in sample solution is less
than the standard solution, the sample will pass the limit test of lead
and vice versa.
Reasons:
Ammonium
citrate, potassium cyanide, hydroxylamine hydrochloride is used to make
pH optimum so interference and influence of other impurities have been
eliminated.
Phenol red is used as indicator to develop the color at the end of process
Lead present as an impurities in the substance, gets separated bye
extracting an alkaline solution with a dithizone extraction solution.
Lead : Click here
Principle:
Limit test of lead is based on the reaction of lead and diphenyl thiocabazone (dithizone) in alkaline solution to form lead dithizone complex which is read in color.
Dithizone is green in color in chloroform and lead-dithizone complex is violet in color, so the resulting color at the end of process is red.
| Test sample | Standard compound |
|---|---|
| A known quantity of sample solution is transferred in a separating funnel | A standard lead solution is prepared equivalent to the amount of lead permitted in the sample under examination |
| Add 6ml of ammonium citrate | Add 6ml of ammonium citrate |
| Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride | Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride |
| Add 2 drops of phenol red | Add 2 drops of phenol red |
| Make solution alkaline by adding ammonia solution. | Make solution alkaline by adding ammonia solution. |
| Extract with 5 ml of dithizone until it becomes green | Extract with 5 ml of dithizone until it becomes green |
| Combine dithizone extracts are shaken for 30 mins with 30 ml of nitric acid and the chloroform layer is discarded | Combine dithizone extracts are shaken for 30 mins with 30 ml of nitric acid and the chloroform layer is discarded |
| To the acid solution add 5 ml of standard dithizone solution | To the acid solution add 5 ml of standard dithizone solution |
| Add 4 ml of ammonium cyanide | Add 4 ml of ammonium cyanide |
| Shake for 30 mins | Shake for 30 mins |
| Observe the color | Observe the color |
The intensity of the color of complex, is depends on the amount of lead in the solution. The color produce in sample solution should not be greater than standard solution. If color produces in sample solution is less than the standard solution, the sample will pass the limit test of lead and vice versa.
Reasons:
Ammonium citrate, potassium cyanide, hydroxylamine hydrochloride is used to make pH optimum so interference and influence of other impurities have been eliminated.
Phenol red is used as indicator to develop the color at the end of process
Lead present as an impurities in the substance, gets separated bye extracting an alkaline solution with a dithizone extraction solution.
Limit Test of Heavy Metals
Principle:
Limit
test of heavy metals is based on the reaction of metallic impurities
with hydrogen sulfide in acidic medium to form brownish colour solution.
Metals that response to this test are lead, mercury, bismuth, arsenic,
antimony, tin, cadmium, silver, copper, and molybdenum. The metallic
impurities in substances are expressed as parts of lead per million
parts of the substance. The usual limit as per Indian Pharmacopoeia is
20 ppm
Procedure:
The Indian Pharmacopoeia has adopted three methods for the limit test of heavy metals.
Method I: Use for the substance which gives clear colorless solution under the specific condition.
Test sample
Standard compound
Solution is prepared as per the monograph and 25 ml is transferred in Nessler’s cylinder
Take 2 ml of standard lead solution and dilute to 25 ml with water
Adjust the pH between 3 to 4 by adding dilute acetic acid ‘Sp’ or dilute ammonia solution ‘Sp’
Adjust the pH between 3 to 4 by adding dilute acetic acid ‘Sp’ or dilute ammonia solution ‘Sp’
Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride
Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride
Dilute with water to 35 ml
Dilute with water to 35 ml
Add freshly prepared 10 ml of hydrogen sulphide solution
Add freshly prepared 10 ml of hydrogen sulphide solution
Dilute with water to 50 ml
Dilute with water to 50 ml
Allow to stand for five minutes
Allow to stand for five minutes
View downwards over a white surface
View downwards over a white surface
Observation:
The
color produce in sample solution should not be greater than standard
solution. If color produces in sample solution is less than the standard
solution, the sample will pass the limit test of heavy metals and vice
versa.
Method II: Use for the substance which do not give clear colorless solution under the specific condition.
Test sample
Standard compound
Weigh specific quantity of test substance, moisten with sulphuric acid and ignite on a low flame till completely charred
Add few drops of nitric acid and heat to 500 °C
Allow to cool and add 4 ml of hydrochloric acid and evaporate to dryness
Moisten the residue with 10 ml of hydrochloric acid and digest for two minutes
Neutralize with ammonia solution and make just acid with acetic acid
Take 2 ml of standard lead solution and dilute to 25 ml with water
Adjust the pH between 3 to 4 and filter if necessary
Adjust the pH between 3 to 4 by adding dilute acetic acid ‘Sp’ or dilute ammonia solution ‘Sp’
Dilute with water to 35 ml
Dilute with water to 35 ml
Add freshly prepared 10 ml of hydrogen sulphide solution
Add freshly prepared 10 ml of hydrogen sulphide solution
Dilute with water to 50 ml
Dilute with water to 50 ml
Allow to stand for five minutes
Allow to stand for five minutes
View downwards over a white surface
View downwards over a white surface
Observation:
The
color produce in sample solution should not be greater than standard
solution. If color produces in sample solution is less than the standard
solution, the sample will pass the limit test of heavy metals and vice
versa.
Method III: Use for the substance which gives clear colorless solution in sodium hydroxide solution.
Test sample
Standard compound
Solution is prepared as per the monograph and 25 ml is transferred in Nessler’s
cylinder or weigh specific amount of substance and dissolve
in 20 ml of water and add 5 ml of dilute sodium hydroxide solution
Take 2 ml of standard lead solution
Make up the volume to 50 ml with water
Add 5 ml of dilute sodium hydroxide solution and make up the volume to 50 ml with water
Add 5 drops of sodium sulphide solution
Add 5 drops of sodium sulphide solution
Mix and set aside for 5 min
Mix and set aside for 5 min
View downwards over a white surface
View downwards over a white surface
Observation:
The
color produce in sample solution should not be greater than standard
solution. If color produces in sample solution is less than the standard
solution, the sample will pass the limit test of heavy metals and vice
versa.
Limit test of heavy metals is based on the reaction of metallic impurities with hydrogen sulfide in acidic medium to form brownish colour solution. Metals that response to this test are lead, mercury, bismuth, arsenic, antimony, tin, cadmium, silver, copper, and molybdenum. The metallic impurities in substances are expressed as parts of lead per million parts of the substance. The usual limit as per Indian Pharmacopoeia is 20 ppm
Procedure:
The Indian Pharmacopoeia has adopted three methods for the limit test of heavy metals.
Method I: Use for the substance which gives clear colorless solution under the specific condition.
| Test sample | Standard compound |
|---|---|
| Solution is prepared as per the monograph and 25 ml is transferred in Nessler’s cylinder | Take 2 ml of standard lead solution and dilute to 25 ml with water |
| Adjust the pH between 3 to 4 by adding dilute acetic acid ‘Sp’ or dilute ammonia solution ‘Sp’ | Adjust the pH between 3 to 4 by adding dilute acetic acid ‘Sp’ or dilute ammonia solution ‘Sp’ |
| Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride | Add 2 ml of potassium cyanide and 2 ml of hydroxylamine hydrochloride |
| Dilute with water to 35 ml | Dilute with water to 35 ml |
| Add freshly prepared 10 ml of hydrogen sulphide solution | Add freshly prepared 10 ml of hydrogen sulphide solution |
| Dilute with water to 50 ml | Dilute with water to 50 ml |
| Allow to stand for five minutes | Allow to stand for five minutes |
| View downwards over a white surface | View downwards over a white surface |
The color produce in sample solution should not be greater than standard solution. If color produces in sample solution is less than the standard solution, the sample will pass the limit test of heavy metals and vice versa.
Method II: Use for the substance which do not give clear colorless solution under the specific condition.
| Test sample | Standard compound |
|---|---|
| Weigh specific quantity of test substance, moisten with sulphuric acid and ignite on a low flame till completely charred Add few drops of nitric acid and heat to 500 °C Allow to cool and add 4 ml of hydrochloric acid and evaporate to dryness Moisten the residue with 10 ml of hydrochloric acid and digest for two minutes Neutralize with ammonia solution and make just acid with acetic acid |
Take 2 ml of standard lead solution and dilute to 25 ml with water |
| Adjust the pH between 3 to 4 and filter if necessary | Adjust the pH between 3 to 4 by adding dilute acetic acid ‘Sp’ or dilute ammonia solution ‘Sp’ |
| Dilute with water to 35 ml | Dilute with water to 35 ml |
| Add freshly prepared 10 ml of hydrogen sulphide solution | Add freshly prepared 10 ml of hydrogen sulphide solution |
| Dilute with water to 50 ml | Dilute with water to 50 ml |
| Allow to stand for five minutes | Allow to stand for five minutes |
| View downwards over a white surface | View downwards over a white surface |
The color produce in sample solution should not be greater than standard solution. If color produces in sample solution is less than the standard solution, the sample will pass the limit test of heavy metals and vice versa.
Method III: Use for the substance which gives clear colorless solution in sodium hydroxide solution.
| Test sample | Standard compound |
|---|---|
| Solution is prepared as per the monograph and 25 ml is transferred in Nessler’s cylinder or weigh specific amount of substance and dissolve in 20 ml of water and add 5 ml of dilute sodium hydroxide solution |
Take 2 ml of standard lead solution |
| Make up the volume to 50 ml with water | Add 5 ml of dilute sodium hydroxide solution and make up the volume to 50 ml with water |
| Add 5 drops of sodium sulphide solution | Add 5 drops of sodium sulphide solution |
| Mix and set aside for 5 min | Mix and set aside for 5 min |
| View downwards over a white surface | View downwards over a white surface |
Observation:
The color produce in sample solution should not be greater than standard solution. If color produces in sample solution is less than the standard solution, the sample will pass the limit test of heavy metals and vice versa.






0 Comments
Please do not enter any spam link in the comment box